Introduction
This tutorial will take you through the basics of Osmosis and some examples of how it applies in physiology.
You will navigate through this tutorial using the buttons at the top of the screen.
The tutorial will ask you questions. Click on your chosen answer to see feedback; click the answer again to make the feedback disappear. When you're finished with one page, click the navigation button for the next page to move ahead.
Have fun! Click on button '1' to see the first page of the tutorial.
Page 1
You learned in chemistry that MOLARITY means the number of moles of a particular compound in a liter of solution.
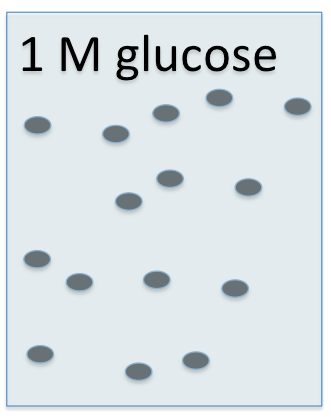
So a solution might be "1 Molar glucose", and it would contain 1 mole of glucose in 1 liter of the solution.
OSMOLARITY is the number of moles of ALL SOLUTE PARTICLES in a solution.
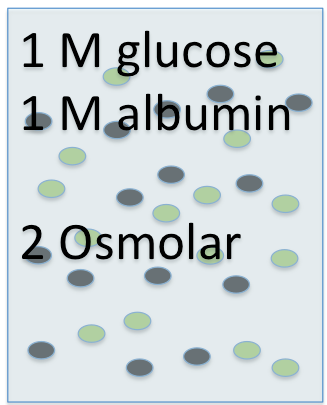
For instance, if you took a 1 molar solution of glucose and added 1 mole of albumin, the solution would still be 1 molar glucose, because there is only 1 mole of glucose in the liter of solution ...
and it would be 1 molar albumin, because there is only 1 mole of albumin in the liter of solution...
but it would contain 2 moles of total solutes, so it would be a 2 OSMOLAR solution.
Page 2
Osmolarity is a very useful concept in physiology, because there are so many different compounds dissolved in the blood that measuring all of them separately is impossible.It is possible to measure the blood osmolarity, though, and that gives an idea of how many total solutes are in the blood.
What we are most interested in, though, is how many solutes are in the blood as compared to the cells, or in one compartment of the body as compared to another.
The reason we care about that is that osmolarity controls the flow of water through cell membranes.
Page 3
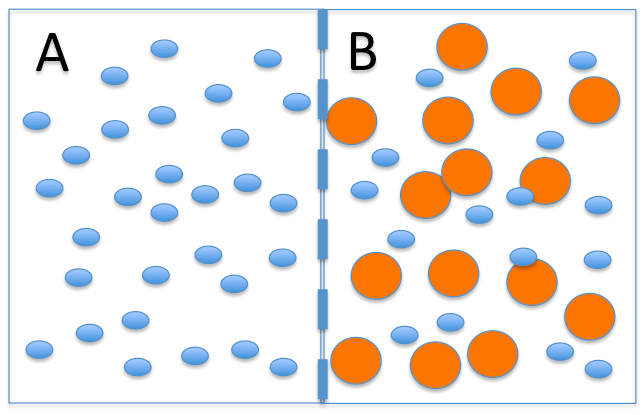
Water can pass through most cell membranes. This means that when a molecule of water hits the membrane, it can go through. The number of water molecules that goes through depends on the number that hit the membrane.Look at the membrane below. Which side of the membrane will be hit by more of the blue water molecules, as they move about at random?
Page 4
More blue water molecules will hit the left side of the membrane simply because there are more blue water molecules on that side of the membrane. Since some of the blue water molecules that hit the membrane can go through, more water molecules will go through the membrane from left to right.This movement of water molecules is called OSMOSIS.

Let's apply some of the terms to this situation. Which of the two solutions has the higher osmolarity?
Which of these solutions do you think would be called the HYPEROSMOLAR one?
Which of these solutions is HYPOSMOLAR?
These terms are comparisons, so you might say one solution is hyposmolar to the other.
Page 5
We've established that a solution with a lot of solutes in it is hyperosmolar and has fewer water molecules than a hyposmolar solution. Since osmolarity depends on the solute concentrations, we're going to stop even drawing the water molecules. In the diagrams below, each dot represents a solute molecule.
Here's a picture of two solutions with different solute concentrations.
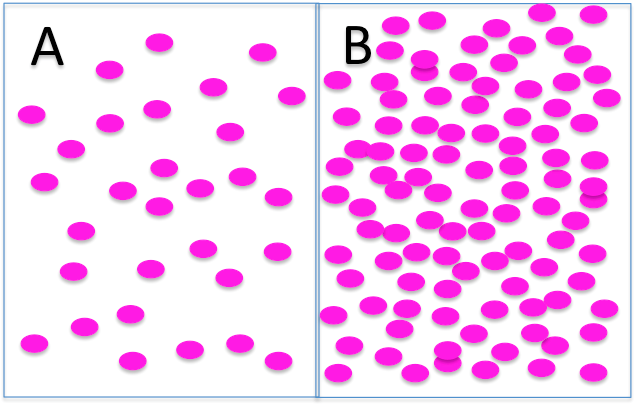
How would you describe the osmolarity of these two solutions?
side A is hyposmolar to side B
Page 6
Try these ones, just to make sure you're comfortable with the terms. Click on the correct word in the sentence below each diagram.
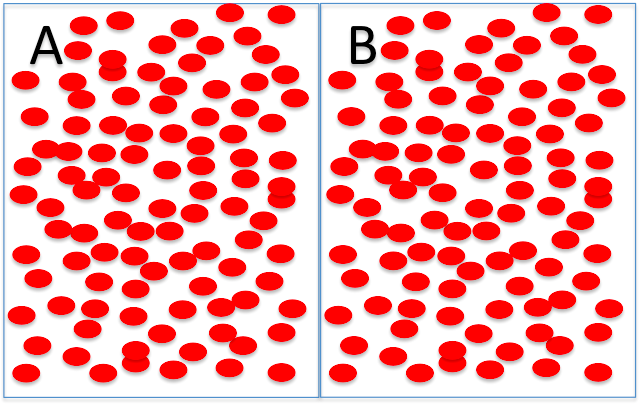
Side A is hyperosmolar/ hyposmolar/ isosmolar to side B
Page 7
I said earlier that osmolarity matters to physiologists because it controls water movement. What is that about?To understand it, we'll need to look back at the first diagram, the one where we drew the water molecules as well as the solute molecules.

Remember, water molecules can cross the membrane if they bump into it as they move randomly around in the solution. The solution with the lower osmolarity, side A, contains more water molecules (blue) than side B, so more water molecules will bump into that side of the membrane and cross it.
That means that more water molecules will cross from side A to side B than will cross from side B to side A. Gradually, WATER WILL MOVE FROM THE HYPOSMOLAR SOLUTION INTO THE HYPEROSMOLAR SOLUTION.
There's a simpler way to remember this:
Water follows solutes.
While this is not entirely accurate, it will work for interpreting most of the situations in basic physiology and pathophysiology.
Page 8
Predict water movement between the solutions. Remember, in these drawings we are not indicating the water molecules - only the solutes.
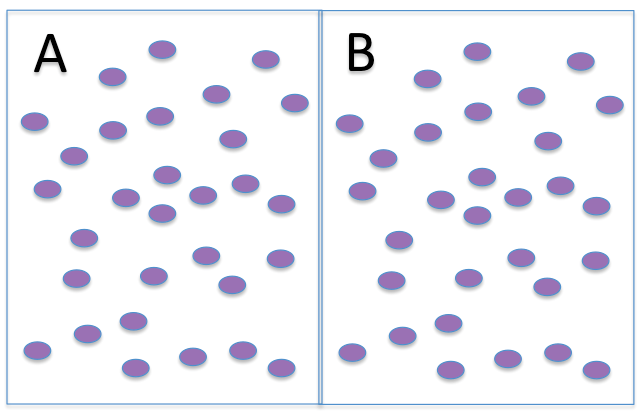
Water will:
- move from side A to side B
- move from side B to side A
- move in both directions equally, for no net movement.
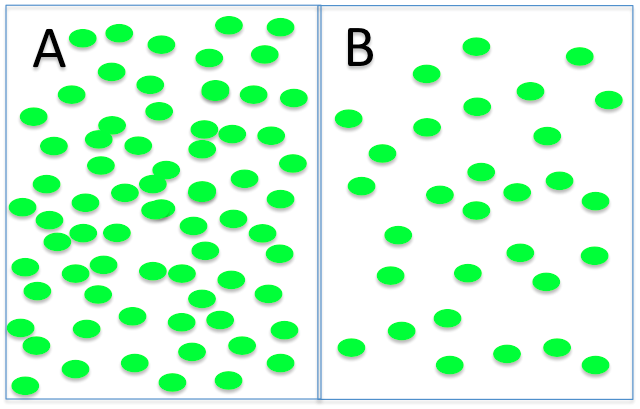
Water will:
- move from side A to side B
- move from side B to side A
- move in both directions equally, for no net movement.
Page 9
Because water is moving into the hyperosmolar solutions, their volume increases. This means that soon they'll be pressing against the walls of their container!
This pressure is called the osmotic pressure. So you might say that a solution is hyperosmolar to the solution next to it, or you might say that it has a higher osmotic pressure; they mean the same thing.
Page 10
Let's apply this to physiology.One of the most important places where osmosis applies to physiology is in the balance between the blood and the cells. Why does this matter? Mainly, because when cells pick up water they swell, and this can interfere with their function. If somebody's brain swells, it can put them into a coma; if their lungs swell too much, they can have trouble breathing. If cells shrink, that can interfere with their function as well.
So here are some cells and the blood surrounding them. You know what to do!
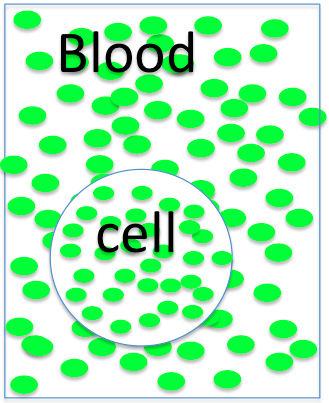
The cell is
- hyperosmotic
- hyposmotic
- isosmotic to the blood, so it will
- swell
- shrink
- not change size.
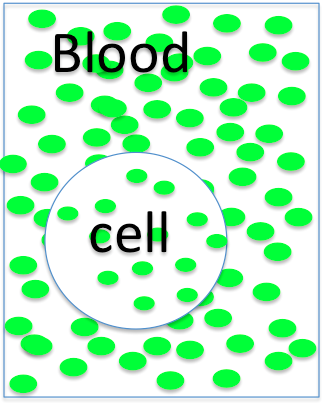
The cell is
- hyperosmotic
- hyposmotic
- isosmotic to the blood, so it will
- swell
- shrink
- not change size.
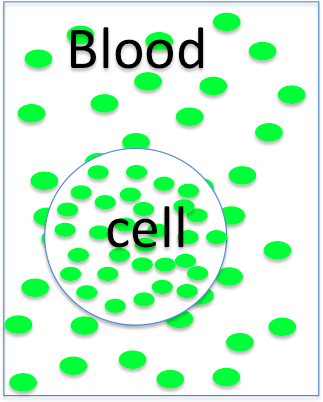
The cell is
- hyperosmotic
- hyposmotic
- isosmotic to the blood, so it will
- swell
- shrink
- not change size.
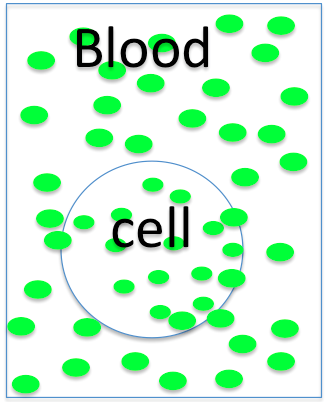
The cell is
- hyperosmotic
- hyposmotic
- isosmotic to the blood, so it will
- swell
- shrink
- not change size.
Page 11
NEW TERM ALERT!
Not all hyperosmotic solutions are equivalent when it comes to cells.
A solution that contains a higher concentration of solutes than the cell does is hyperosmotic, that's true -- but it won't matter if those solutes can just leak into the cell and even out their concentration. So when we consider osmosis in physiology, we're interested in the solutes that don't leak into the cell easily.
Don't worry - you don't need to sort the solutes out yourself! We have chemists for that. But we do need a special term to talk about the concentration of those solutes:
TONICITY
So when we're talking about physiology, instead of a hyperosmotic solution, we use a hypertonic solution. Instead of hyposmotic, we use hypotonic, and instead of isosmotic, we use isotonic.
Page 12
A man drinks six gallons of distilled water, which is absorbed into his blood. Which of the diagrams below best represents his situation? Click on the figure label to check your answer.
Adding the water to his blood made it become hypertonic / hypotonic to the cells. That means the cells were hypertonic / hypotonic to the blood. Water moved from the blood into the cells / from the cells into the blood , making the cells shrink / swell .
Page 13
A man develops a kidney disease which makes him lose lots of the proteins from his blood in his urine. (Hint: proteins are solutes!)Which of the diagrams below best represents his situation? Click on the figure label to check your answer.
Removing the solutes from his blood made it become hypertonic / hypotonic to the cells. That means the cells were hypertonic / hypotonic to the blood. Water moved from the blood into the cells / from the cells into the blood , making the cells shrink / swell .
Page 14
A woman was given a hypertonic IV by mistake in the hospital. (Hint: an IV is a solution added directly to the patient's blood)Which of the diagrams below best represents her situation? Click on the figure label to check your answer.
Adding the solutes to her blood made it become hypertonic / hypotonic to the cells. That means the cells were hypertonic / hypotonic to the blood. Water moved from the blood into the cells / from the cells into the blood , making the cells shrink / swell .
Extra thought: Do you think the woman needs to drink or urinate to get herself back to normal?
Page 15
The problems you just solved had to do with people whose blood composition changed, so their water balance between cells and blood was altered. But osmosis can also control water movement into and out of other spaces in the body. The GI tract is a perfect example.A woman just ate a big meal and filled her GI tract up with food (solutes).
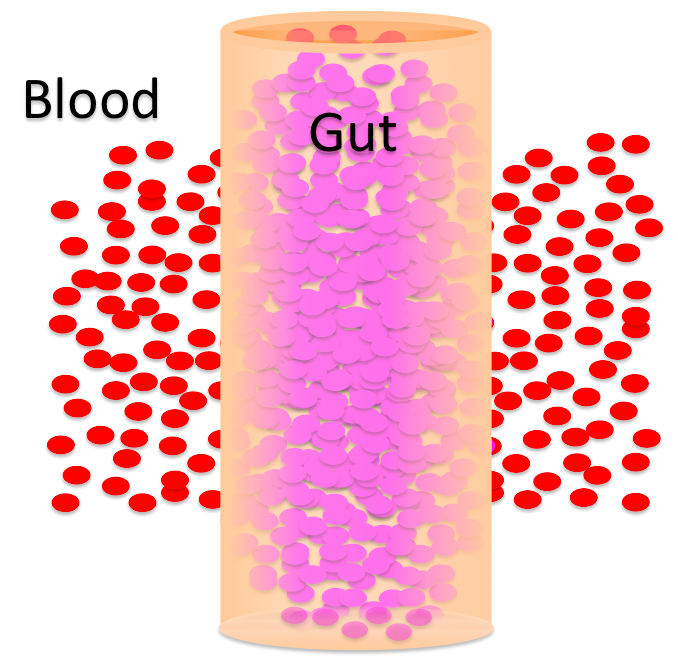
The contents of her intestines are hypertonic / hypotonic to her blood. That means the blood is hypertonic / hypotonic to the intestinal contents. Water will move from the blood into the intestinal contents / from the intestinal contents into the blood .
Page 16
This looks like a problem, doesn't it? If you lost water from your blood into your intestines every time you ate, it could mean big trouble for you. Let's follow this food a little further, though. Further down in her intestines, the woman's cells begin to absorb the food. They move those solutes from her intestines into her blood.
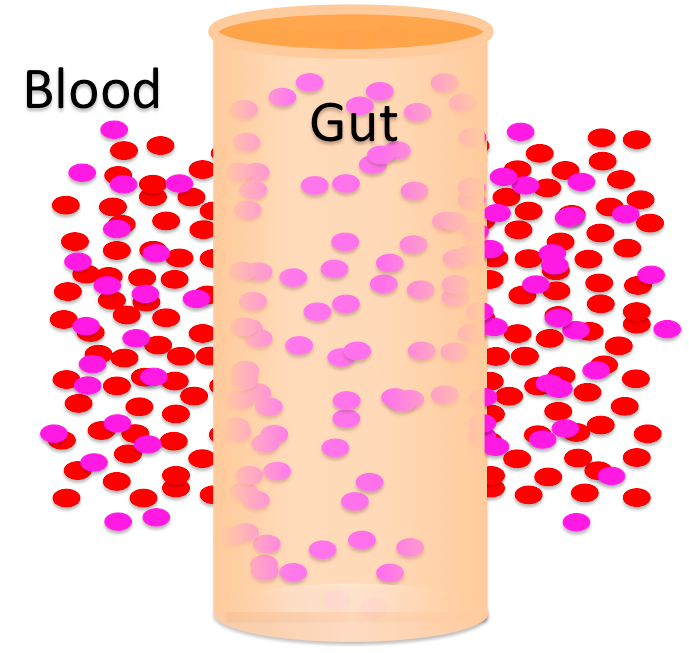
As solutes move from her intestines into her blood, the contents of her intestines become hypertonic / hypotonic to her blood. That means the blood is becoming hypertonic / hypotonic to the intestinal contents. Water will move from the blood into the intestinal contents / from the intestinal contents into the blood .
This example shows you something new about osmosis.
Not only does WATER FOLLOW SOLUTES into the blood or the cells or the intestinal contents: WATER FOLLOWS SOLUTES when they move from one area to another.
So if you want to move water from one part of your body into another, the way to do it is to move solutes. Then the water will follow them. Water doesn't care which solutes it follows; it always follows the majority of solutes.
Let's look at some situations in which your body uses this trick to make water go where it needs to.
Page 17
Here's a tubule in your kidneys. The tubule is full of urine, and it has an ion pump called the Na+/K+ ATPase, that moves ions (solutes) between your blood and your urine. Your kidneys turn this pump on or off, depending on whether you need to keep water in your body or get rid of it in your urine. Let's figure out how it works!
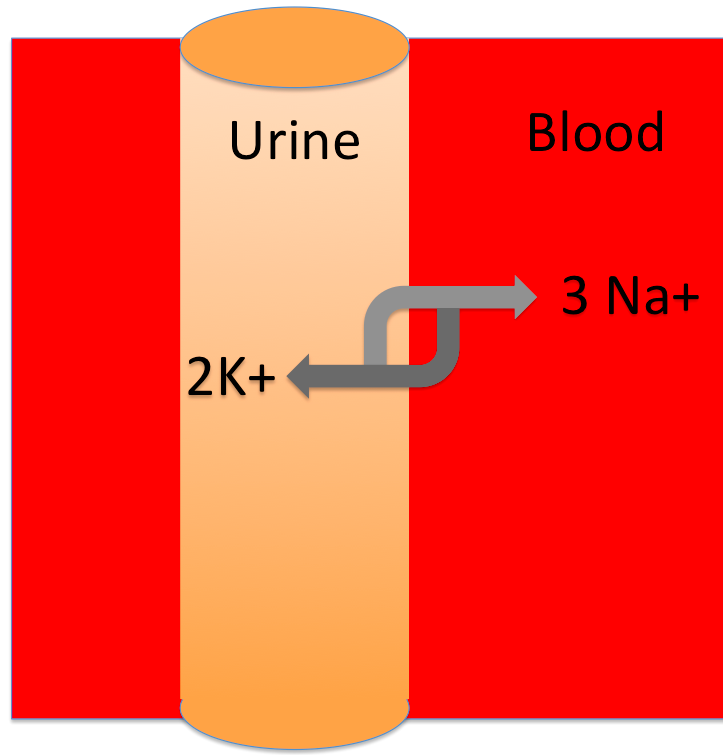
When this pump runs, it will move more solutes into the blood / urine . That means the blood is becoming hypertonic / hypotonic to the urine. Water will move from the blood into the urine / from the urine into the blood .
Page 18
Your individual cells can use the same trick. Because cells have to contain lots of enzymes and other molecules, they are actually usually hypertonic to the blood. Plus, Na+ is always leaking into them from the blood, and that adds even more solutes. But cells can't afford to swell up - it could damage or kill them! So they, too, have the Na+/K+ ATPase.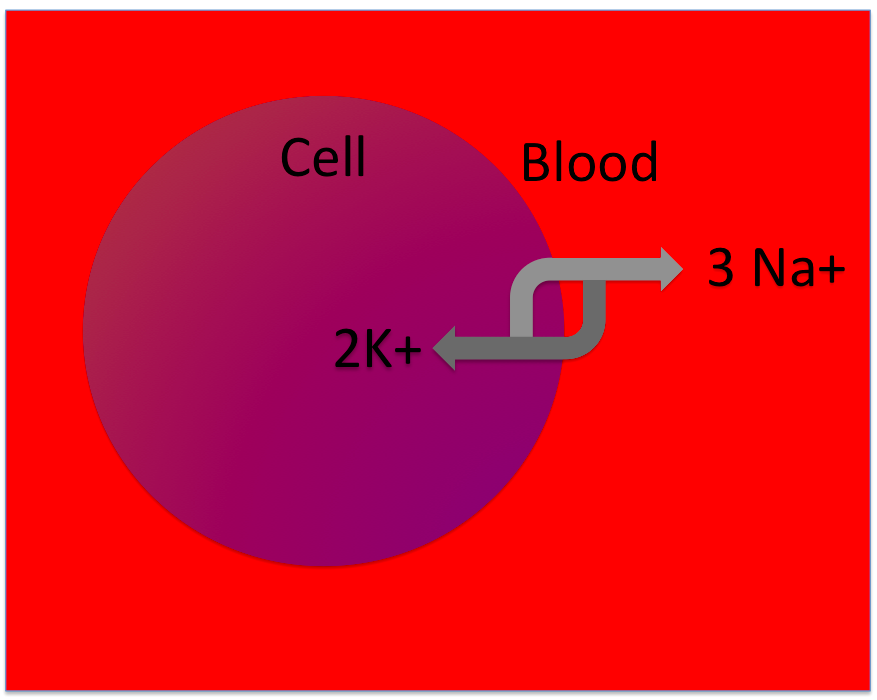
When this pump runs, it will move more solutes into the blood / cell . That means the blood becomes hypertonic / hypotonic to the cell. Water will move from the blood into the cell / from the cell into the blood .
Extra thought: the Na+/K+ ATPase requires ATP, or energy, to run. If the cell couldn't produce enough ATP, would it shrink or swell ?
Congratulations on finishing the tutorial on osmosis. You should be able to apply it now to figure out where water will go in any situation in which there is a solute imbalance. You can approach any osmosis problem with the method you saw on these practice problems:
2.Find which solution is hypertonic and which is hypotonic
3. Predict the direction of water flow
4. Infer the consequences.
Good luck and have fun!
Sources used in creating this tutorial include:
Porth, C., 2002. Pathophysiology. 6th Edition. Lippincott, Philadelphia, Pa.
Seeley, R.R. , Stephens, T.D., and P. Tate, 2003.Anatomy and Physiology, 6th Edition. McGraw-Hill Higher Education.