This tutorial will take you through carbon dioxide production, transport, and removal from the body. It will also address acid-base balance and respiratory acidosis/alkalosis.
You can navigate through this tutorial using the buttons at the top of the screen.
The tutorial will ask you questions. Click on your chosen answer to see feedback; click the answer again to make the feedback disappear. When you're finished with one page, click the navigation button for the next page to move ahead.
Have fun! Click on button '1' to see the first page of the tutorial.
Page 1
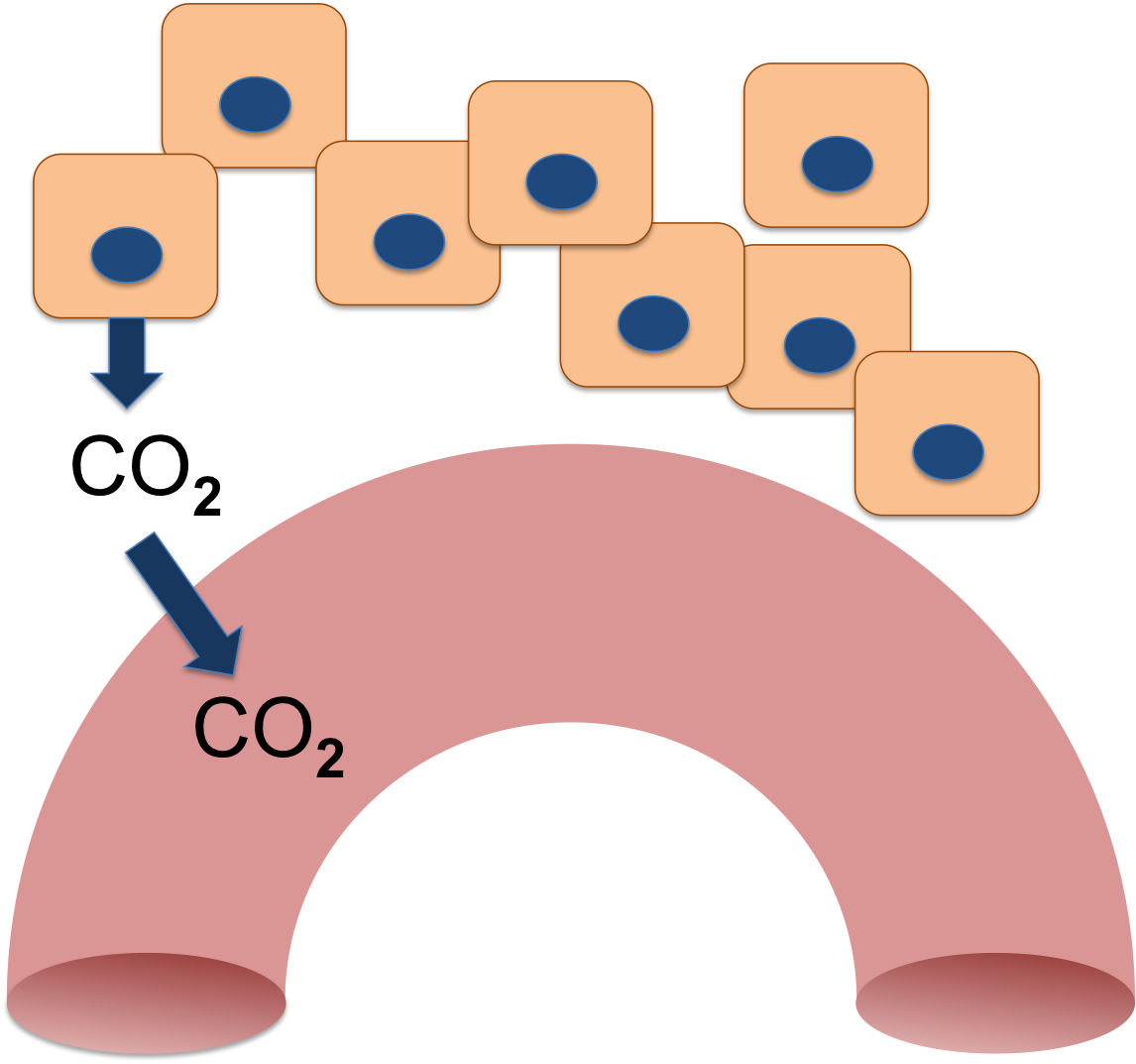
Your cells produce Carbon Dioxide (CO2) as an end product of aerobic respiration. When molecules are broken down in the Krebs cycle, CO2 and water are made.
The CO2 diffuses out of the cells and eventually enters the blood flowing through the tissues. The blood carries it away.
Because it is a gas, the CO2 that is dissolved in the blood is called the partial pressure of CO2, or the pCO2. Like all physiological pressures, it is measured in mm Hg. Your blood normally has a pCO2 between 35 and 45 mm Hg.
Page 2
pCO2 = CO2 dissolved in the blood
However,CO2 is always being produced by your cells. They can produce up to 2500 mL of CO2 per minute when you are exercising hard - but blood with a normal pCO2 will only be carrying 2-3 mL of CO2 in each 100 mL of blood (Arthurs and Sudakhar, 2005).
How can your blood carry all that extra CO2?
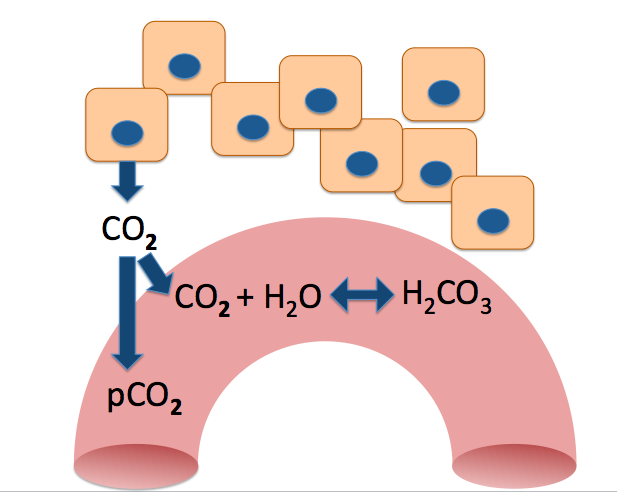
Let's zoom in to the capillary and see what's going on in there.
You can see that some of the CO2 is combining with water to form a new compound. This is H2CO3 , or carbonic acid.
Arthurs, G. J., & Sudhakar, M. (2005). Carbon dioxide transport. Continuing Education in Anaesthesia, Critical Care & Pain, 5(6), 207–210. http://ceaccp.oxfordjournals.org/content/5/6/207.full'
Page 3
Most of the CO2 in your blood undergoes this reaction, becoming carbonic acid. While the reaction can happen in your plasma, it happens much faster inside your red blood cells, because they contain an enzyme, carbonic anhydrase, that catalyzes it.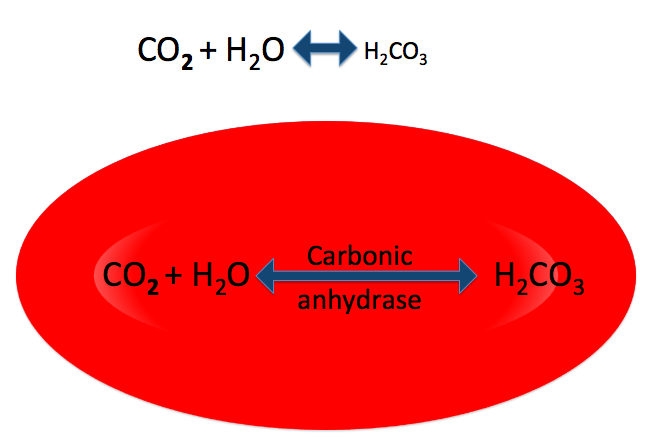
Once the CO2 has been converted into carbonic acid, the carbonic acid will dissociate into H+ and HCO3-, or bicarbonate anion.
H+ is the ion that lowers pH, making the blood acidic. So the more CO2 you have in your blood, the more H+ will be produced and the more acidic your blood will become.
Page 4
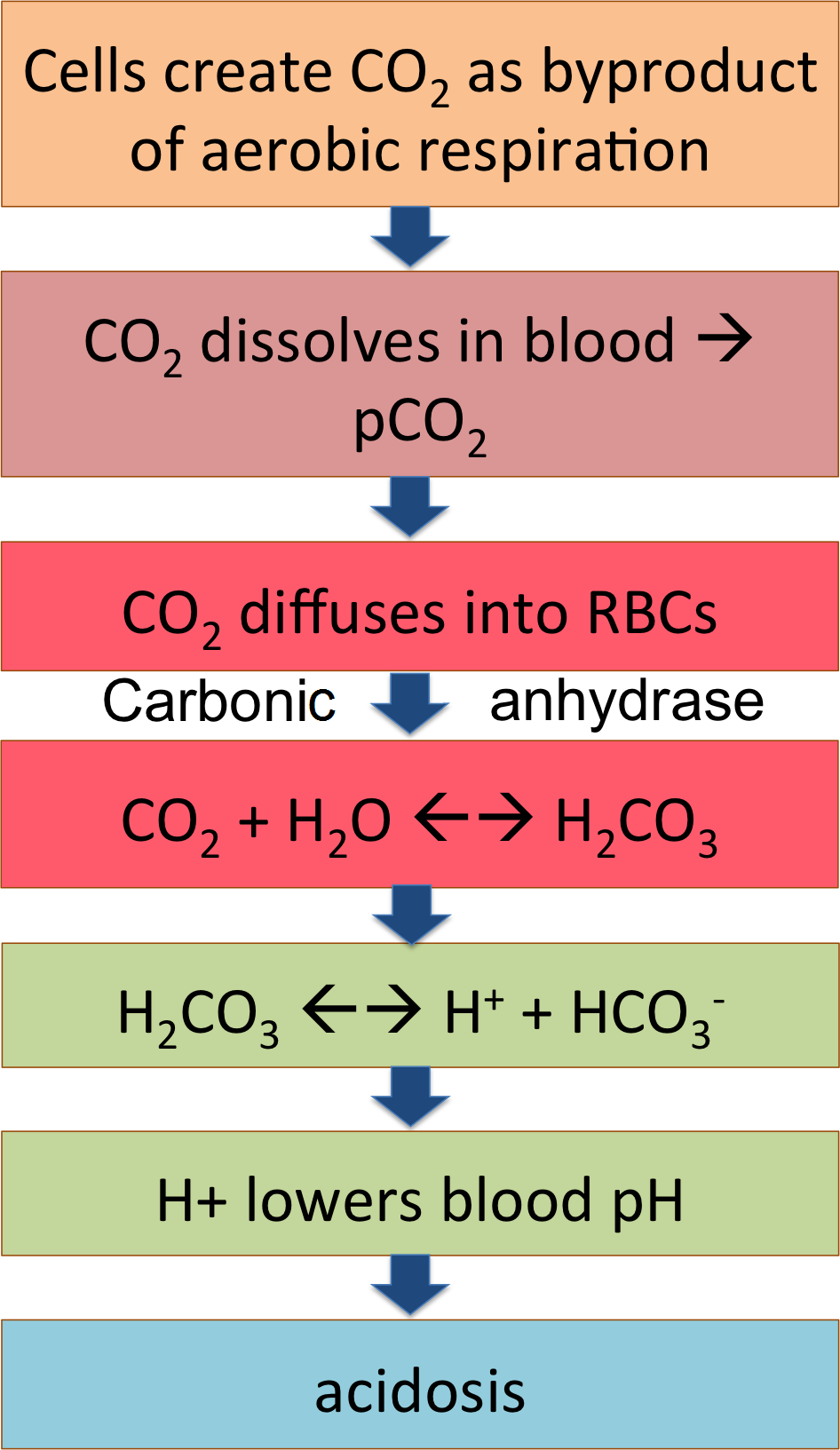
The flow chart summarizes this pathway; CO2 from the cells becoming carbonic acid in the blood and dissociating to release H+, lowering blood pH. That's what happens out in the tissues, loading the systemic venous blood with CO2 and acid.
What do you think will happen in this blood when it reaches the lungs?
The blood will release the H+ into the air in the lungs
Nothing much, because the lungs load the blood with O2, not CO2.
The pathway will go backwards and turn the acid back into CO2 and H2O.
If someone breathes too rapidly (hyperventilates), what will happen to blood pH?
It will decrease as acid levels decrease
It will increase as acid levels decrease
It won't change, because it's CO2 being exhaled, not acid
Suppose someone's blood was too acidic. Would this person need to breathe faster or slower to fix the blood pH?
Page 5
A man is having an asthma attack, and can't exhale enough air. He's having difficulty breathing; his blood pCO2 is elevated and H+ levels are high as well.
What term best describes his situation?
The man complains that he just can't catch his breath. His face is flushed and warm. What is giving him this breathless feeling?
Page 6
A man is having an asthma attack, and can't exhale enough air though he is trying hard to breathe. His blood pCO2 is elevated and H+ levels are high as well. He has respiratory acidosis.
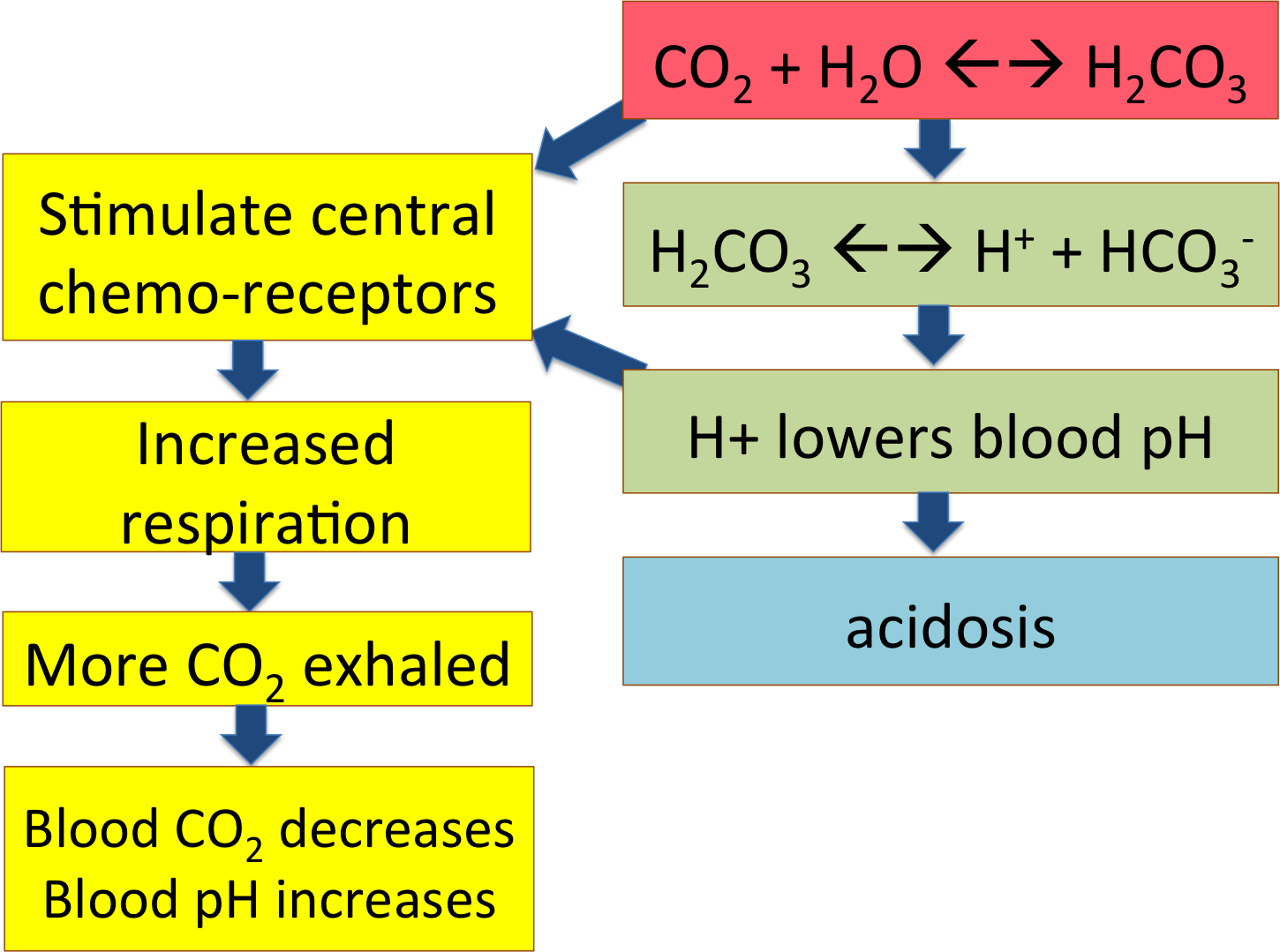
The high pCO2 is diffusing into his cerebrospinal fluid and becoming carbonic acid, leading to low pH. This is affecting the central chemoreceptors in his brain, telling him he needs to breathe more to exhale the excess CO2 and bring his blood pH back up to normal.
But now his face has gotten redder, and his eyes are bloodshot. He complains of an awful headache. What could be causing this?
What would make this happen? If you think back to the nervous system unit, can you remember any effect H+ has on nerve and muscle cells?
Page 7
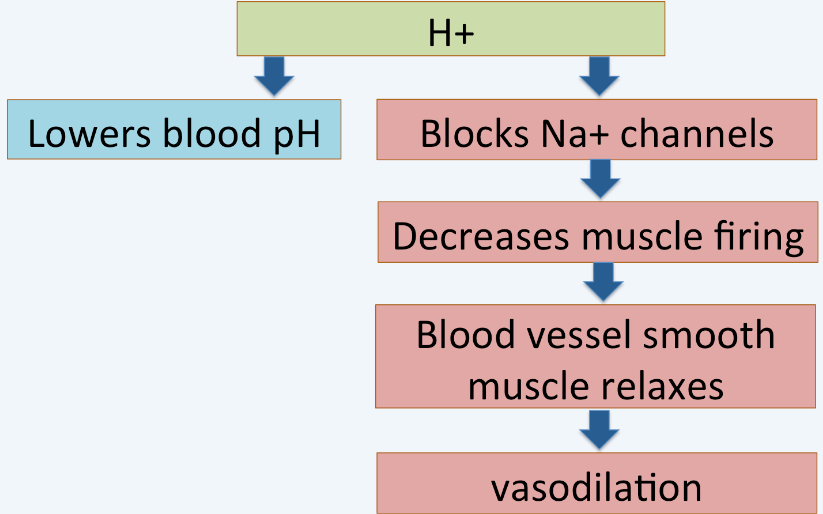
Did you remember it? H+ ions block the Na+ channels that let Na+ enter cells so they can fire. This man is flushed because the smooth muscles in his blood vessels can't fire enough, or contract enough - so his blood vessels are dilating.
What will happen to his blood pressure?
Page 8
A second man has come in to the clinic with chronic bronchitis.
He has the same high pCO2 and low pH as the first man - but he isn't breathing heavily at all. And he's not flushed, but blue!
What explains the difference?
The answer is in his central chemoreceptors. This man has a chronic pulmonary disease, which means that he has had difficulty breathing for a while. His pCO2 has been elevated and his pH has been low for months, maybe years -- and his central chemoreceptors have become habituated or used to this, the way you get used to a bad smell in a room. They just don't notice any more!
Page 9
If his central chemoreceptors are no longer keeping track of his pCO2 and pH, though, what is keeping him breathing at all?
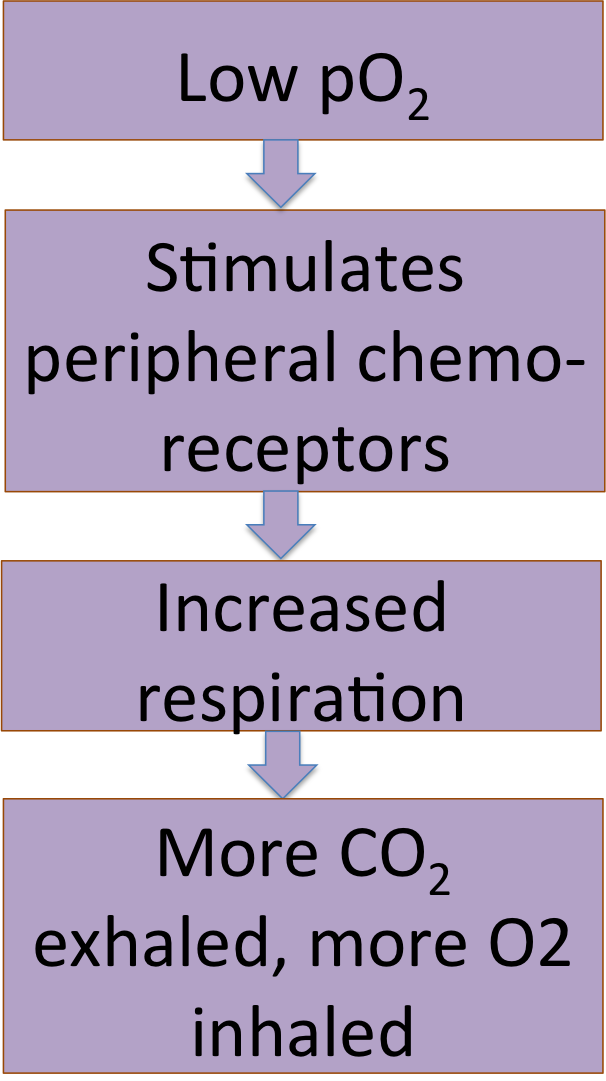
He has a second set of chemoreceptors, the peripheral chemoreceptors. These are located in the carotid arteries and aorta, and they are affected by both pCO2, pH, and pO2. These chemoreceptors will tell him to breathe when he starts to run out of O2.
The client with chronic bronchitis and a blue coloring (cyanosis) was given a pulse oximetry test which showed his hemoglobin was 90% saturated with Oxygen, too low. The doctor ordered low-flow Oxygen to raise his Oxygen saturation.
Everyone working with the man was warned not to turn up his Oxygen.
Why shouldn't it be turned up?
High Oxygen would stop his breathing
High Oxygen would lower his pH
This is the end of the tutorial on CO2 metabolism. Happy studying!